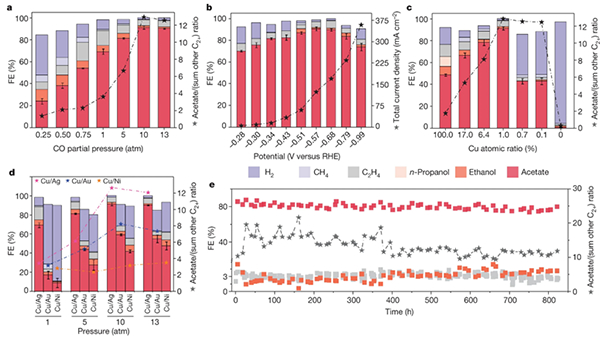
Fig. Performance of CO-to-acetate electroreduction using Cu/Ag diluted alloy catalysts
With the support from the National Natural Science Foundation of China (Grant No. 52006085), Prof. Yuanjie Pang and Associate Prof. Jian Jin from Huazhong University of Science and Technology built a high-pressure electrocatalytic carbon monoxide reduction device. By combining Cu-in-Ag dilute (about 1 atomic percent of Cu) alloy catalyst with a high-pressure flow-electrolyzer, the researchers have achieved an acetate Faradaic efficiency of 91%, an energy efficiency of 34% and a stability of 820 hours, all record-breaking in CO electroreduction. The research results were published in Nature on May 3, 2023, with the title "Constrained C2 adsorbate orientation enables CO-to-acetate electroreduction" (Nature.2023, 617: 724–729. full article link at http://www.nature.com/articles/s41586-023-05918-8.)
Electrochemical reduction of CO to value-added chemicals, the critical step in the cascade carbon dioxide electrolysis, offers a route to the long-term storage of renewable electricity that enable the transition to carbon neutrality. Copper is the only known element that can grant electrocatalysts with the ability to produce major amount of multi-carbon molecules, but it also suffers low selectivity when it comes to targeting one from over twenty possible products.
Having decided to bring the selectivity of acetate to near-unity in CO reduction, the research team saught to stabilize the key intermediate of acetate formation, the monodentically bound *C=C=O group on an atomically small copper site dispersed in a non-copper host. However, the excessively small copper sites need higher reactant molecular coverage to complete C-C coupling. The research team designed a high-pressure flow-electrolyzer device, which enriched the reactant molecular coverage on the catalyst surface. By optimizing the design of the device flow channel, the pressure resistance inside the reaction device was greatly reduced, thereby ensuring the stability of the reaction interface. At the same time, the high pressure can accelerate the material transport rate at the reaction interface, thus reduce the energy barrier in the reaction process, and achieve the purpose of improving the selectivity of acetate products and the energy efficiency of the system. In this work, the selectivity of acetate is as high as 91%, and it can still be maintained above 80% in a wide potential window. In addition, the selectivity can keep more than 80% for more than 820 hours in a membrane electrode assembly electrolyzer device, and the energy efficiency of the system is as high as 34%. Techno-economic analyses show that operating at elevated pressure adds low dedicated capital and operating costs, which ensures the application prospect of this technology in the future.
This work has realized a sustainable process, which converts carbon dioxide into acetate through carbon monoxide in two steps by using renewable electricity, and has realized zero-carbon green production of acetate, and achieved high selectivity and high energy efficiency. Importantly, this work has confirmed the application potential of carbon dioxide electrolysis technology in distributed clean energy storage, and the feasibility of green synthesis of carbon-based chemicals using carbon dioxide electrocatalytic conversion technology.

Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@donnasnhdiary.org
京ICP备05002826号 文保网安备1101080035号 Copyright 2017 NSFC, All Right Reserved