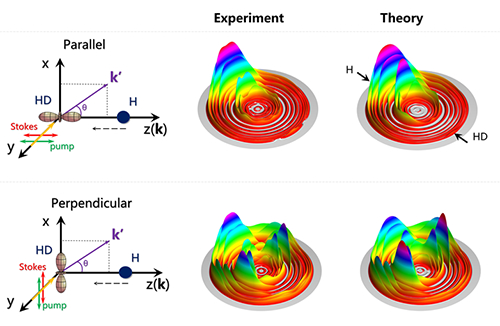
Figure. The schematic of two collision geometries and corresponding results. Lasers are used to control the direction of the HD bond, and the H atom collides with the HD molecule in parallel or perpendicular geometries. At the collision energy of 0.50 eV, the differential cross sections of the H+HD→H2+D reaction of two geometries show notable differences.
Supported by the National Natural Science Foundation of China (Grant Nos. 22288201, 22173097, 41827801, 22103084), Prof. Chunlei Xiao, Prof. Zhaojun Zhang, Prof. Donghui Zhang, and Prof. Xueming Yang’s research team from the Dalian Institute of Chemical Physics have realized stereodynamical control of chemical reactions by controlling the direction of the chemical bond. The research result was published in Science titled "Stereodynamical control of the H + HD → H2 + D reaction through HD reagent alignment" on January 13, 2023. Article link: http://www.science.org/doi/10.1126/science.ade7471.
The nature of the chemical reaction is the collision of atoms and molecules which results in the breaking and forming of chemical bonds. The stereodynamical effects, which focus on the influence of the spatial orientation of reactant molecules on the reaction process during collision, is a fundamental and important issue in the chemical reaction. The stereodynamical effects come from the fact that molecules have particular shapes and thus can not be simply treated as mass points during a chemical reaction. For example, a hydrogen molecule, which looks like a dumbbell, is formed by two hydrogen atoms connected with a covalent bond. When another reagent collides with a hydrogen molecule, it can attack either one of the hydrogen atoms or the covalent bond. Consequently, the dynamical process and reaction probability of these two situations may have obvious differences. It has long been a frontier issue to take advantage of the stereodynamical effect to accurately control chemical reactions in the research of chemical dynamics.
Researchers used the stimulated Raman pumping technique to efficiently prepare hydrogen molecules to a specified rovibrational excited state and align the hydrogen bond axis simutaneously. They measured the results of the H+HD→H2+D reaction in two collision geometries at collision energies of 0.50, 1.20 and 2.07 eV, and observed notable differences in the angular and quantum-state distribution of the produced H2 (Figure). Nonadiabatic quantum dynamical simulation accurately reproduced the experimental observations. The stereodynamical effects was further uncovered and analysed in detail through the combination of dynamical simulation and the polarization-dependent differential cross sections, revealing that quantum interference plays an important role in the reaction with the perpendicular configuration.
With the combination of the high-resolution experiments and the accurate quantum dynamical calculation, this work demonstrates that the control of the chemical reaction is feasible by controlling the alignment of hydrogen molecules, and indicates that our understanding and the ability to control chemical reactions have reached a new level.

Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@donnasnhdiary.org
京ICP备05002826号 文保网安备1101080035号 Copyright 2017 NSFC, All Right Reserved