Supported by National Natural Science Foundation of China (Grant No.52090020, 91963203), the research team led by Prof. Zhisheng Zhao from Yanshan University, in collaboration with domestic and oversea scholars, elucidated the direct phase transformation from graphite to diamond under static high pressure, which has remained elusive for more than half a century. At the same time, they discovered a new class of hybrid carbon materials. The result entitled “Coherent interfaces Govern direct transformation from Graphite to Diamond” was published online in Nature on July 6, 2022. Website link: http://www.nature.com/articles/s41586-022-04863-2.
Graphite and diamond are the most common carbon materials in nature. The direct transformation from graphite to diamond usually occurs in a “black box” of high pressure and high temperature (HPHT), and understanding the transformation mechanism has remained a significant challenge ever since the first successful static synthesis of diamond. With state-of-the-art scanning transmission electron microscopy (STEM), the research team observed and determined the coherent interfaces between graphite and diamond nanodomains for the first time in partially transformed graphite samples treated under static HPHT, and elucidated the graphite-diamond transformation mechanism: The graphite layers are locally bonded into coherent interface with two rhomboid and two rectangular structural motifs, the coherent interface then advances to the graphite region and transforms graphite into diamond. The various combinations of the four structural motifs produced a variety of coherent interface structures, leading to rich substructures (stacking faults, twins, diamond polytypes, etc.) in the transformed diamond regions. This new solid-solid phase transformation mechanism, different from the classical nucleation and growth mechanism as well as concerted shear mechanism, may work for other covalent materials, such as IVA elements and IIIA-VA compounds.
The research team termed the hybrid carbon material with coherent graphite-diamond interfaces as Gradia. Gradia possesses an excellent combination of mechanical and electrical properties. Depending on the proportions of graphite and diamond in Gradia, Knoop hardness is adjustable between 51 and 115 GPa; room-temperature electrical resistivity is adjustable between 8´10-4 and 4.9´105 Ω·m; fracture toughness is so high that it cannot be measured with the indentation method. With combined properties advantages of graphite and diamond that are tunable by changing the proportions of graphite and diamond, Gradia provides opportunities in pursuing desired combination of mechanical and electronic properties, such as simultaneous superhardness, high toughness and electrical conductivity(Figure).
The direct transformation mechanism from graphite to diamond revealed in this work enriches the category of solid-solid phase transformation. Beyond the transformation mechanism, the newly discovered hybrid carbon material, Gradia, marks a major step towards nanostructure and properties engineering in diamond-related materials, and provides insight in developing next-generation high-performance materials.
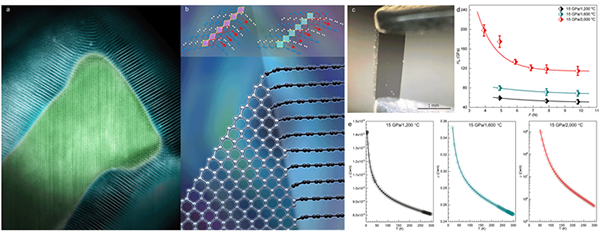
Figure (a) Atomic resolution STEM of graphite-diamond interfaces (the graphite layers are emphasized with white lines); (b) Schematic diagram of coherent graphite-diamond interface (inset: selected structural motifs); (c) A Gradia sample; (d) Knoop hardness of different Gradia samples; e, Electrical resistivity of different Gradia samples.

Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@donnasnhdiary.org
京ICP备05002826号 文保网安备1101080035号 Copyright 2017 NSFC, All Right Reserved