Supported by the National Natural Science Fund (Nos: 21925404, 22021001, 21775127, and 21991151), the research teams led by Professor Jianfeng Li from Xiamen University and Professor Feng Pan from Shenzhen Graduate School, Peking University have made major progress in understanding the nature of interfacial water. The research was published in Nature on December 2, 2021, entitled “In situ Raman spectroscopy reveals the structure and dissociation of interfacial water”. The article's link is http://www.nature.com/articles/s41586-021-04068-z
Water molecules play a vital role in various domains of science. The comprehension and management of water molecules at the electrode/solution interface lay the foundation for progress in surface science, catalysis and energy science. Especially, water molecules directly participate in many important electrocatalytic reactions, such as hydrogen evolution reaction (HER), oxygen evolution reaction (OER), carbon dioxide reduction reaction (CO2RR), oxygen reduction reaction (ORR), nitrogen reduction reaction (NRR), etc. The core of electrocatalytic processes is the structural evolution of interfacial water as a function of applied potential, which necessitates in-situ characterization of water molecules at electrode surface (especially atomically flat single crystal surface), and more importantly, a realistic model of the corresponding structure-activity relationship in electrocatalysis. However, interfacial water is notoriously difficult to probe owing to the interference from bulk water and the complexity of interfacial environments.
Based on the shell-isolated nanoparticle-enhanced Raman spectroscopy(SHINERS) technology invented by the research team (Nature, 2010, 464, 392-395), this work achieved the in-situ monitoring of the structure and dissociation of interfacial water at Palladium single crystal electrode/solution interface during HER process. The authors found that the interfacial water molecules were constituted by both the hydrogen-bonded water and the cation hydrated water. The cooperation of cations and negative potential assigns an ordering to the interfacial water, such that the cation hydrated species are dragged closer to the electrode surface than the hydrogen-bonded counterpart. This scenario improves the charge transfer efficiency between the interfacial water molecules and the electrode surface, thus greatly boosting the HER rate. A higher concentration or valence state of the cations will impose an increased degree of ordering to the water molecules at the interface and further improve the HER performance. The central role of cation hydrated water was also substantiated by the intimate correlation between the content of cation hydrated water and HER rate on various facets and with different electronic structures, implicating that such rationale can be generalized to other material systems.
The observation and analysis of interfacial water structure have opened up an avenue to explore the mechanism of surface reactions and manipulate the water chemistry for targeted application. With a wealth of experimental evidence and theoretical simulations, the research team found that cations in aqueous solutions can effectively tune the catalytic efficiency via the "co-catalytic" effect. As a consequence, the composition and structure of electrolyte solution (such as cation concentration and valence state of hydration) and solid-phase interface structure can jointly exert their influence on the catalytic reactions, which is a kind of co-catalytic effect. This work provides a robust and adaptive strategy for improving the electrocatalytic reaction rate.
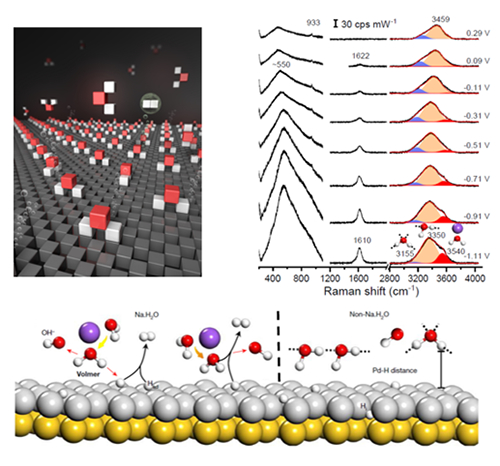
Figure. The in-situ Raman spectra and dissociation process of interfacial water.

Add: 83 Shuangqing Rd., Haidian District, Beijing, China
Postcode: 100085
Tel: 86-10-62327001
Fax: 86-10-62327004
E-mail: bic@donnasnhdiary.org
京ICP备05002826号 文保网安备1101080035号 Copyright 2017 NSFC, All Right Reserved